Leading Minds Network Report: BioPharma Supply Chain Data Innovation
The above word cloud is feedback during a live polling of participants of the 2019 Leading Minds New Jersey seminar, answering the question “What themes do you want to hear at this seminar?”.
INNOVATION is on the minds of all practitioners in temperature control life sciences distribution. The promise of technology is exciting. The possibility of saving millions in bad shipments is irresistible.
Over two days, practitioners exchanged ideas and their experiences across topics including:
-
Stability data in the Supply Chain
-
Continuous Monitoring vs. Risk-Based Approaches
-
IoT and Wireless Technology
-
ELPRO’s View on Data Innovation
About Leading Minds Seminars
Leading Minds Seminars are largely discussion-based, unique in format and intent: we call it FUELS (Fusion of Useful Experiences in Logistics and Storage). Learn about upcoming seminars in your area.
Stability data in the supply chain
Moderated by Geoff Glauser, with input from Tom Scalone and audience.
The group agrees that using stability budgets is inevitable but even more so important in order to save cost and improve efficiency in the process. One of the major challenges is to push forward the remaining stability downstream in the supply chain. The group have addressed the following challenges:
-
How do we assess the stability budget of a specific product in a specific shipment? Does the system know the stability budget of my product?
-
If you have a consolidated shipment with many products in the same container monitored by one data logger: how do you assess each product separately based on the same monitor data?
-
No matter if (or if not) we had a deviation in the first shipment leg - how do you transport the remaining stability budget to the following transport legs? Often you don't know which pallet has been where before - so how do you refer to the remaining stability budget?

All of this is a software challenge and the group agrees that it can only be solved by integrating the cold chain database with the ERP (typically SAP).
The discussion continued and new questions came up if local authorities are accepting the use of stability budgets, including:
-
Health Canada has very strict rules and regulations. They might ask critical questions on using stability budgets vs. shipping on strict label conditions. But the experience of the group is that with the right data, risk analysis and rationale you can convince them and they accept using the stability data.
-
EU GDP has (compared to the US/FDA) very clear rules. There are documentation requirements that needs to be fulfilled but they are clear and therefore easy to fulfil.
-
Brazil obviously has very high requirements - sometimes absurdly high. But the experience shows that if you push back on the requirements by asking questions "how exactly do you mean this" - and they often lower their requirements.
One important take out from the stability budget discussion with regulators is to have a realistic view on the sensitivity of your product. A 2-8°C product for example, which is not sensitive to freezing is much better shipped at a lower temperature setting than at strict 2-8°C (since exposure to heat is much more dangerous). By doing so, you might be able to safe significant money (e.g. by using low cost dry-ice cooling containers vs. expensive "strict 2-8°C packaging"). Key lesson: Make sure to include freeze/thaw cycles into your stability data early in the process.
Continuous Monitoring vs. Risk-Based Approaches
Presentation and discussion led by Lisa Moher.
Lisa has showed an innovative system how to perform local US shipments in a validated process and container without adding temperature monitors to each single container. The process includes a qualified box based on seasonal temperature profiles (origin & destination) which is used in a validated process using a daily weather forecast email for the operations personnel to decide on the packaging. The entire process is validated regularly by qualification shipments proofing that the process works - in these qualification shipments, data loggers are added to a minimal load and the data is collected in a database. FDA has audited the process and did not have major findings. So, Sanofi is using this process today for all local shipments. Lisa explains the cost benefit as follows:
-
In the year 2000 Sanofi has 1 Mio. local shipments and added chemical indicators to each box for 1$. The problem at the time was that chemical indicators were not reliable (e.g. nurses at hospitals but the indicators to an oven and still the color did not change to alarm...) so the customers lost confidence.
-
2019 Sanofi ships 3 Mio. local boxes. Adding an electronic device at cost of 3$ would mean a 9 Mio. $ budget which is not acceptable for Sanofi since they are mainly cost driven.
Feedback from audience was extremely positive. However, some pointed out that ‘not monitoring’ is not possible for higher value shipments, for example losing one shipment could result in higher lost product value than the 9 Mio $ spent on data loggers. Lisa advised this is the same case for US, where non-last mile shipments are shipped at label claim and monitored.
Another consideration is the "no monitoring" process works perfectly in domestic last-mile but it does not work on intercontinental shipments, on high-risk lanes and if the product needs to be re-shipped, as pointed out by Lisa.
One of the discussions then taking place was about the load in qualification shipments. The group agrees that every additional product box added to a container increases the thermal mass and therefore minimum load is the worst case you should test in every qualification shipment. An empty shipment is the "worst case of minimum load" and therefore probably the safest bet.
IoT and Wireless Technology
Discussions led by: Gordon Johnson, Jeroen van Loo, Bill McGillian, Martin Peter
It’s clear, the industry is moving to real-time monitoring. Or actually is it wireless or "hands free" technology that is needed? These are the discussions the group at Leading Minds had.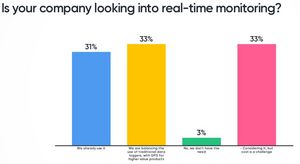
What problems can IoT solve?
-
Capture data from any device and recommend relevant information
-
Location/temperature monitoring
-
Automated data retrieval, no human interaction needed
-
Cost / risk reduction & process improvement > This balance is tricky!
-
Unique/custom applications
-
Data sharing
-
Predictive modeling
What problems could IoT create?
-
Losing experience by overreliance on “IoT” – young people not being trained properly on the “why” something is being done
-
Data ownership issues could pose a problem
-
Data integrity is a hot topic – companies are being “hit” in audits
Group’s conclusions and key learning points
-
Just adding cost and collecting (real-time) information will not work. Although the large pharma companies are not willing to add extra cost for more expensive devices across all shipments, in fact the group is well aware that adding real-time capabilities adds significant extra cost. Clear to everybody that the cost will come down over time. So bottom line is: at the moment this new technology will be used for high-risk & high-value shipments until the cost come down to be applied more broadly.
-
The value of IoT must be clear and defined along the triangle of increasing quality, improving on time and/or lowering cost. So, the benefit of IoT must be determined in the specific shipment process.
-
The expectation is that real-time data is used and processed in three ways:
Automate Processes:
- automated upload
- automated stop by geo-fence
- automated assessment
- automated releaseManual Analysis
- Position data is there in case somebody wants to analyse manually:
- in case of a deviation (where did it happen)
- in case of an insurance case (who's fault was it)
- for risk management (heat map: where do deviations happen most often)
- for packaging engineering (does my box work? do I need to improve or is there room for cost savings?)
Opportunity to intervene:
- temperature excursions on the way
- deviation from geo-fence/routing
The ultimate dream
Following discussions on requirements, limitations and realities, the group discussed what would the ultimate solution be in the future, in terms of hardware and overall wishes. They included:
-
Box-level (or even vial-level) indicator covering the entire supply chain from filling to patient. Device knows the stability and can tell OK/ALARM (e.g. by LED or on an Epaper). Eventually it would also show the expiry date and adjust dynamically depending on the temperature history.
-
The device is communicating its status once per day and the Software could alert/alarm:
> if a large amount of stability budget has been used up (something went wrong -> excursion happened)
> if it left the "usual route" (e.g. based on historic data: where did similar boxes move before)
> if a box has been opened -
Data is more value than the money. Big pharma so big they have multiple forwarders, a data monitoring partner, several packaging partners – how is all that data combined, streamlined so analytics can be done. Some looking at how to integrate these different sets in the cloud.
However, the group is well aware that at the moment this ultimate dream is not possible due to cost, size and battery. "But who knows what will be possible in 5 years?"
ELPRO’s View on Data Innovation
Today ELPRO uses various wireless technologies to automated processes and upload data directly to the database to achieve “contactless real-time monitoring”, including Bluetooth and GSM devices.
But the “intelligence” isn’t just in the hardware. Rather it’s in the software that uses sophisticated workflows that adapt to specific situations, or specific products for release under certain conditions. For example, consider 5 different drugs, each with a different set of stability budget. How is the remaining stability budget inherited to the next transport step? The software needs to be organized in a way that monitors, assesses and releases products; not shipments.
For last miles, especially high value products e.g. biologics, personalized medicines, gene therapies, Direct to Patient, etc. – LIBERO ITS has been a valuable solution that is small enough, cheap enough, thin enough and smart enough to go sales unit level and monitors the entire supply chain from filling to patient. Read more about LIBERO ITS here including technical specifications and solution video. Or if you are in clinical supply, don’t miss the white paper describing use cases of LIBERO ITS for clinical supply chain Direct to Patient distribution. ELPRO and Sonoco ThermoSafe were delighted to host such an interesting meeting of the minds, collaborative seminar.
Some impressions of the Leading Minds Seminar New Jersey 2019







Leave a Comment