Expert Q&A: Cold Chain & Distribution Contingencies During COVID-19
On Tuesday May 19th, a live webinar was held to discuss supply chain and distribution center contingencies during the CV-19 pandemic. This article outlines the Q&A discussion had with QA distribution experts:

/csm_Pers_Michael_Holloweck__27810207c7.jpg?width=166&name=csm_Pers_Michael_Holloweck__27810207c7.jpg)
Patrick Girten, Manager, Quality Distribution US/PR, Bristol Myers Squibb | Michael Hollweck, Senior Manager QA Logistics Americas, Celgene
Topics discussed:
-
Precautionary DC operations during COVID
-
Creating redundancy in distribution with secondary sites and logistics providers
-
Approving new contingency routes
-
Virtual audits – is this a viable future for pharmaceutical QA supply chains?
(The opinions expressed during the webinar and in the following article are solely those of the presenter(s) and not necessarily those of Bristol-Myers Squibb Company. Bristol-Myers Squibb Company does not guarantee the accuracy or reliability of the information provided. The information provided is given in summary and Q&A form and does not purport to be accurate or complete, and should not be considered as advice or a recommendation. Before acting on any information, you should consider the appropriateness of the information, and you should seek independent advice.)
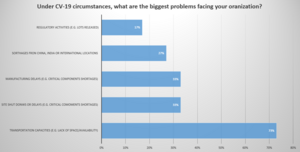
Image 1.1: Audience Poll Question
Moderator: Supply chains across industries all over the world are broken and faltering. Pharmaceutical supply chains face the same challenges, with one big difference – the patient. While many pharmaceutical businesses are fighting to supply kits and find treatments and vaccines for COVID-19 – there are still the patients with life-threatening illnesses or chronic diseases that need their medications.
This webinar will talk with a panel of QA and logistics experts about their experiences during COVID-19 in re-routing, re-planning their distribution center operations and supply chains. We will discuss contingency plans, current impact, and how they foresee business post-COVID.
Q: What are some of the precautions your DCs are taking?
Patrick: We are doing what you hear everybody else on the news doing; masks, gloves, social distancing, staggering shifts. But these can create challenges that we wouldn't normally see because we’re spreading the workforce out and just not as efficient as we used to be so it creates challenges. We also had to approve a disinfectant protocol. We had to go through our Environmental Health and Safety and approve the chemicals that are needed and not just the chemicals that are in the United States. It's the chemicals that are approved worldwide. Remember the recall for TBA that Johnson & Johnson had to recall with wood pallets? Bleach was actually something they recommended you stay away from. So in the current situation, you know, we know bleach is typically what you want to use. So we had to look at other chemicals. So all of those provisions we looked at early on.
Mike: For Celgene most of what we deal with is our third parties. So we have third party Logistics providers and the responsibility is theirs but it's our partnership with them to identify exactly like Patrick had said it's identifying where they do what. PPE are they using how are they controlling one of the big issues that we had in the state of Pennsylvania in the U.S. If there was a requirement by the state that all of the trucker's had to wear masks. So they had to have some kind of a face mask. They were driving in and when they were unloading and loading trailers. So the question came up. Okay. How do we manage the truckers who do not have a mask… how do we manage that load/unload. Do they send the truck to have somebody else deliver it as the person go and get a mask, but it's trying to make sure that you understand not just like Patrick was okay with saying which is how do we manage with our own sites? And how do we manage our products but also taking a look at the local and state laws that may impact our business and managing with our partners to try to identify.
How do we make sure that the product is flowing? And how do we make sure that the supply chain is still intact while obviously making sure that our people and our third parties people being safe and that actually we were talking about this earlier and that brought up a good discussion with our third party where we were talking about as a distribution center. We discussed, what happens if you have a trucker where they have a temperature. They said, “we don’t let him in”. Then, how do you get the load in?
There are DoT rules around dropping the load and another tractor hooking up, or a different driver delivering, or a different company delivering. How do you do that when you have a truck with products sitting on the dock, so it made us think step through those plans. It sounds good on paper, but actually try to do it and then talk to those companies that you're dealing. Explain to them - here's what our plan is, and open up communication – a big factor.
Moderator Question: Could you foresee any circumstances where a DC could be shut down completely?
Mike: Yes it’s a possibility especially since most of our distribution comes through third party and you definitely have that possibility of having a breakout of COVID in a distribution center where it's not our management team making decisions. Or it could be local health inspector or County ordinance with specific legal requirements that forces a shut down, or they deem the risk too high. That's why we run through our business continuity plans often and execute them at least every six months. If one of our distribution centers gets closed for any particular reason such as COVID outbreak, we would absolutely be able to satisfy the business needs dealing with our other two locations. So can it be shut down? I'll tell you right now with COVID, I am not doubting anything bad that can happen with our supply chain right now so I could absolutely see that and that's where business continuity comes in.
Patrick: Absolutely, same here, we have multiple DC's so built for business continuity.
“Good business continuity plans step through all the possibilities, poke holes in the ‘what if’ scenarios and try to make them fail.” Patrick Girten, BMS
Question: Do you exercise drills for these ‘what if’ scenarios?
Mike: I think that's part of the challenge that we've been seeing with COVID. As you’re executing the business continuity plans, you might not identify a specific reason for the Distribution Center shutting down… it could a fire, tornado, tsunami …anything could be closing your distribution center. But that's not what you're identifying; rather the discussion is now that I don’t have this distribution center, I don’t have the product available, how do I manage that? How do I flow it through the rest of my supply chain through my other distribution network. How do I manage that?
The ‘what if’ scenarios are part of the questions asked during business continuity planning for this type of business. Such as earlier Patrick was saying, what if trucker comes in with a temperature, how do you manage that? I think in some cases, it's also a really good thing to have people who have extra teams on site for people who are thinking ahead of these type of issues. They can help identify if there is a problem when dealing with a corrective action. Then ask, what's causing that issue or how do I look and see what if this takes place? What are potential issues do a failure mode effects analysis and try to identify what would take place once it has failed.
Yes, you're running around trying to ship product out while you're doing these ‘what if’ scenarios, but as Patrick said it's better to be able to come up with ahead of time. Consider beforehand, what do I do when a trucker turns up with a high temperature, vs. when the guy is sitting at your door?
Patrick: We also do the routine business continuity drills, but one of the things I think is not to focus on the ‘What’ but rather the ‘How’. So you don't say what if we have a pandemic or what if we have this.. rather it’s what happens WHEN something X happens, what will we do? Talking through having the right people in place, and of course communication is a huge piece. Where are the procedures for you BCP? On a company computer or company website? What happens when everybody runs out of the building because there's a fire and you leave your computers there. Well, okay, so we stuck one at the guard gate in a paper file. Hey, that's great. What happens when your home gets a call that you know something happened. So good business continuity plans step through all the possibilities, poke holes in the ‘what if’ scenarios and try to make them fail.
Moderator Question: What supply chains have been hit the hardest for you during COVID?
Patrick: For us at BMS, it’s the processes that have been in place and just chugged along with no problem for many years. For example, samples management. There's not been many changes to that process, but in COVID with the entire face to face missing, the whole process broke down. Then you have to then come up with a new way to manage that.
Mike: We also faced challenges with our sales samples. We overlooked in our business continuity plan the fact we had one single supplier. We should have asked ourselves, if we’re dealing with a state on potential lock down and facility is shut. All of sudden, we were dealing with not being able to get professional samples out. Then we were faced with - is there a way to quickly on board another site for this same provider in Delaware and still a way that satisfies all of our needs. Many things like this changed dramatically overnight.
With single sourced supply chain for sales samples, BMS and Celgene identified next steps:
Shipping Direct to Practitioner
-
DTP was already established but rarely used
-
Use has been ramped up significantly to make sure that samples are available
Business Continuity
-
Need to establish a second site
-
Same provider that we use has a facility in Delaware
-
Worked to identify what steps needed to have Delaware Facility as an approved supplier
-
Determination for virtual audit of facility
“It was just amazing how quickly things were failing that you did not expect.”
Mike: It was just amazing how quickly things were failing that you did not expect. So for example, pretty much a lot of our products are shipping from the EU here into the U.S. The manufacturing sites are in the EU and we would we would ship a lot of our product through JFK and Newark. All of a sudden the flight was canceled or you would only have one flight a day and every single Oracle and different types of companies are trying to get their products on one flight. Then you add the fact that some of the worst places that were hit in the entire world are the states of New Jersey and New York, with mortality rates similar to Italy. So the governors of both of those states were starting to impose some very strict regulations and making sure of people going to work and probably lock in, you know shelter in place rules.
How do you manage something that that did not even seem realistic six months ago. If I told anyone that we would not be able to get air freight coming into Newark or JFK people would have looked at me strange. But quickly we realized the question really was, what are the alternate routes? Is there a way to fly through Dulles? Is there a different carrier?
Or another huge solution from the carrier’s was, well if we can’t transport people, we do have capability to ship goods. So they took out seats in the passenger jets, and made it possible to ship goods where people would normally be sitting. The business changed for the airlines where they can actually ship goods, if not people.
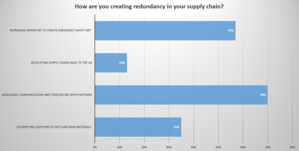
Image 1.2: Audience Poll Question
Moderator Question: What were some of the activities that you did for temperature sensitive products, how did you know from a quality perspective they were ok?
Mike: There's a couple pieces to that. First, for all our transportation lanes, we have a risk assessment that documents the details out exactly how to determine actual risk to that product in that lane.
At Celgene, our risk assessment includes:
1-9 severity scale
-
5 different risk criteria
-
Average maximum temperature
-
Average Minimum Temperature
-
Duration of the route
-
Product Risk category
-
Route complexity (multi-modal or multi-stop)
Each risk criteria has a different weight. Final weighted score is the risk score.
So for example, if the shipment is going from Phoenix Arizona here in the United States, a desert, to Toronto Canada, which as you know, a very cold location because it's Canada. So an average maximum temperature is going to be different for Toronto. The first criteria is the average maximum, then the same thing with the other criteria of average minimum temperature. So we're looking at the maximum and minimum throughout the seasons time of the route. So how long is the duration? What's the duration of the route?
For duration, if shipping from my location here in New Jersey, to our location in Pennsylvania, it's maybe two or three hours. But if I'm shipping from my location in New Jersey and I'm shipping it into Australia - obviously that's a much longer. You got to get a longer flight. You've got a much more longer route. So what's the length of the route.
Then what is the product risk category. Different products have different stabilities; they might have a higher risk when there's a temperature excursion. For example, if it’s a +2 °C to +8 °C product, it might have a higher risk category than a tablet that we make.
The last piece is route complexity. If it’s a two hour truck from NJ to PA, vs. multi-modal going to Australia – that is going to make a big difference to calculating risk.
So when there is a change, like with COVID, instead of going into Newark, it would into Dulles Washington DC, what’s the change on the right assessment then with factoring in the 5 new criteria? In an emergent situation, it’s also doing the new risk assessment very quickly.
What happens when a new supplier is not already on the approved supplier list? How quickly will you QA protocols allow them to be vetted and added? Usually it takes about 3 months. Clearly we can’t take that long or we’re dead in the water. So we have to figure out how to do that in a in a quicker way while maintaining our integrity, according to procedure and according to our defined processes.
That’s been another huge impact of COVID. We’ve been critical on our processes, identifying why something that normally take an long extended process – why? Why does is it take so long? How do I try to tighten that timeline? Is it because my colleague said it takes weeks to review a deviation?
Patrick: In the beginning of COVID, when we would open an Emergency Change Control, we ensured ‘COVID’ goes in the title, so later on we can easily identify them and consider for future contingency planning. We can go back and say what all did we do? Because you have a lot of different business units doing a lot of different things to make this happen. So if they all put ‘COVID’ in the title, you can go then and do a search in our system for ‘COVID’ to go back later on.
Mike: One of the key aspects of communication was we literally set up daily conference calls at 8am where we had add every single organization globally that would go through and we had an organizer that just literally said, okay EU operations. Talk to me. Tell me what's going on. What issues are you seeing? What's good, what’s bad? How about Asia PAC, Mexico Latin America… every single group had the opportunity to go through and explain and say here’s where I need help. We created a global community and a Global Network of knowledge that share expertise, and resources if possible, within the distribution Network. Conversations would include Finance as well to ensure shipments go through.
For example, someone says ‘I've got a shipment that hasn't delivered yet and it's going to be real challenge to get it to Australia by Friday”.
We learned quickly it was imperative to establish daily global cooperation and communication during COVID with all departments and regions, including the right leadership to own the communication streams and expedite decisions.
Initially it started out as multiple calls, one for transportation, one for logistics. But in the end we realized get everybody on one call and let’s see if that works better.
Patrick: For BMS as well, it was important to have a cross departmental call regularly. All of a sudden, we were faced with new things like getting product OUT of the country. So we needed customs on these calls. We would solve one problem and move to the next, as a global team.
COVID Internal Communication Lessons:
We started daily calls when it became clear that we could see issues that could impact delivery to patients and customers.
-
All impacted groups (ops, finance, transportation, QA)
-
Across all regions globally
-
Upfront and honest discussion – requests for help, direction on where the current issues stand, potential issues
-
Minutes from the meeting with specific actionable items
-
Daily calls have been scaled back to weekly calls
Moderator Question: What has been your experience so far with Virtual Audits?
Patrick: During COVID, we had to stand up a these other operations pretty quickly, and it was all virtual. In this case virtual means just questionnaires and phone calls. It’s not live video like this webinar for an hour.
Usually we’d be on-site for a full day doing an audit. Could you imagine trying to do this, live video, for an entire day? For some of our GMP audits, they can last 3, 4, 5 days. So it’s definitely an interesting topic how we will do this in the future. When you’re sitting in an audit room, you can get a feel for how things are going. There's a mood in the room, a tempo and face to face interaction.
However, virtual audits may play better into the younger generation. My kids are used to sitting on Xbox and hearing 30 or 40 people in their ear at the same time and somehow they manage through that.
I suspect in the future, we won’t be traveling as much to audits, we’re learning that now. But I believe there are some things you just have to be there in person.
Moderator Question: Are there any parts of a partner’s process that you would be concerned with validating by video or by documentation alone?
Patrick: Distribution is different than GMP. For distribution facilities, there are variables such as do they store non-pharma products, are they a cross dock? One of the things that I can't do virtually is smell. At a cross dock, you can smell the different things that you know are leaking or if a generator is puffing oil and gas. In a virtual audit those could be overcome with questions such as; “What are the products that go through your facility?”, or “What spill control measures do you have in place?”.
As we discussed before, you have to think through it again and again. What sounds good on paper doesn't always work when you step through it. Ask “If I was there, what would I feel, smell or touch? How am I going to get that same result?”
Mike: It’s exactly like Patrick was saying… if I do not have that ability to come into a distribution center, how can I see if there is a lot of product that's sitting on the floor, or it’s not very clean, or an odor. You lose that in a ‘virtual audit’.
For example with deviations, how do you look at batch management or how do you look at their systems system validation if they're using some kind of a system or how do you look at their security processes? Sure you can look at a document that says, we're going to lock all of our doors at night. It’s how do you get that comfort level?
Virtual audits could be a huge challenge; I'm used to having that personal interaction. I'm used to that I need to have put my eyes on something to be able to see it and to make sure that you know, you telling me that you're keeping the place clean is not the same as me going out and seeing that you're keeping the place clean. I mean if I ask you and say hey Patrick, are you keeping your Warehouse clean? What's your answer going to be? Of course they will say yes.
-
Virtual Audit Recommendations
-
Establish documented requirements
-
Establish minimum documents checked
-
Establish an audit checklist for what needs to be captured
-
Ask for specific documents and areas for control. The audit is more of a “I ask for evidence, they provide and I review”
-
A virtual audit is difficult because you cannot get a feel for the client. You cannot see what is causing a problem. You cannot gauge where the audit is going or what is causing an issue
-
Recommend having a specific time where you speak with the auditee so you can get a feel and answer your questions (think of it like an interview)
Bonus off-line question Moderator: What do you see as forever changed post COVID-19 for pharmaceutical supply chains?
Patrick: People are creatures of habit, our memories are short, pocketbooks are tight, processes have evolved over years of scrutiny, change is hard and businesses are built to fulfil a need. If during this time we discover more efficient ways of doing something or see gaps that only a world-wide pandemic can uncover then there will be some change, but for the most part I think things will go back to the way they were. Since every industry is different, I think the common good outcome will be that we now have another question to ask ourselves when making a decision. What if? That might lead to less travel, more technology, etc. BUT, there is always a but, what are the unintended consequences of changing established processes? If the business was a balanced before this, take a second look before making sweeping decisions. I live in the county and used to get rid of the snakes...so now I have lots of mice which can chew through wiring and burn my house down (almost happened). Ooops, I only saw the problem in front of me and upset the balance of nature. I hope we don't fool ourselves into thinking that since the company kept operating this new way that all of those old processes need to be changed.
Mike: I see more robust supply chains and alternate routes in the event that a route has been taken away. Once we have assessed the new routes they can be used in the future if needed bad results - I think the impact to specific industries like the airlines will be huge. That will lead to decreased service and increased costs. Also, I think that the distribution center model has held up pretty well but that will be looked at to see how to prevent outbreaks at sites. I think there was more impacted sites than people will recognize and that may come out in the future.
About the autors:
Patrick Girten, Manager, Quality Distribution US/PR, Bristol Myers Squibb. Patrick works at Bristol-Myers Squibb in Manufacturing and QA Compliance roles since 10 years. The last 10 years as Manager of Distribution Center QA US/PR. In his position he is responsible for ensuring compliance in BMS storage, transportation and distribution of pharmaceutical drug prducts. He reviews/approves HQ Impact Assessments, Global Packaging Technology and Global Logistics packaging assessments, lane and thermal package qualifications/protocols, Quality Agreements, SOPs, Change Controls, Temperature Excursions. Patrick has a BS in Aeronautical Technology from Purdue and AS in Aviation Maintenance. He also is a volunteer firefighter, rescue scuba diver, NASCAR pit fireman and hydroplane dock diver. Patrick is located in Mount Vernom, Indiana.
Michael Hollweck, Senior Manager QA Logistics Americas Celgene. He is currently QA for logistics for Americas for Bristol Myers Squibb (Celgene). Celgene is a pharmaceutical manufacturer with a global distribution network and main internal manufacturing facilities in Switzerland, US (Arizona) and also external manufacturers around the globe (China, Germany, US) with current product lines span multiple shipping temperature requirements (CRT, +2 °C to +8 °C, -20 °C, -70 °C) and involve products throughout the product lifecycle (DS, DP). Michael got experience in distribution quality across multiple companies (J&J, Celgene, BMS) and across multiple product types (Pharm, med device, consumer). He is located in Rockaway, New Yersey.
Stay informed about topics like this and more affecting the pharmaceutical supply chain. Subscribe to the Leading Minds Network and join us at an upcoming webinar or seminar.







Leave a Comment