Stability Budget 101: Q&A with Leading Industry Practitioners
Can stability data really save the life of your products, and prevent millions of dollar loss to your company?
At the end of a complex pharmaceutical supply chain, a product has seen many forms, labs, cities, containers, boxes, docks, trucks, storage facilities, data loggers and finally one patient. If the product is temperature controlled, how do you know it’s been kept in correct temperature and quality conditions from source of APIs, to manufacture, through multiple legs of distribution to patient? Many companies today haven’t asked themselves this question yet. But the regulators are.
With both European and Unites States Pharmacopeia drafting new guidance on GDP for APIs in 2015, it’s clear the pharmaceutical regulatory landscape is moving toward complete visibility of medicines from raw ingredients through manufacturer, all the way to the patient. Not only are they asking for evidence of temperature control throughout the product lifecycle; they are looking into the integrity of how the data is collected, stored and used for risk-based decisions. Go in-depth on the GDP for APIs subject by reading the white paper GDP for APIs New Regulations.
So how do you track excursions along multiple legs of an end-to-end regulated complex supply chain? The answer is… stability budget.
What is a stability budget?
Whether you currently use it or not, as a pharmaceutical manufacturer you have a stability budget for products. Through numerous types of stability studies, the Pharma Development team establishes a stability profile for each product. The stability budget combines relevant information from temperature studies with available data from the stability testing to determine the amount of time a product can spend out of its labelled storage conditions without risk to its quality, safety or efficacy.
As a product moves through its lifecycle from API through multiple legs in a supply chain; part of the stability ‘budget’ is used in each ‘leg’ to determine at the end of the lifecycle whether it’s safe to be consumed, or not. By proactively setting up and managing a stability budget, you solve the issue of QA running around doing manual investigations with CROs and LSPs for multiple legs of a shipment taking days, sometimes weeks. Quicker decisions at the end of shipment avoids delays to patient administration, and keeps the supply chain in control.
So is stability data a super hero?
Truth be told. When you speak with pharmaceutical manufacturers, there are actually not that many examples of having to throw out millions of dollars of products (however those cases do exist!). But that’s not to say they didn’t spend days if not weeks trying to ‘save’ the products by manually retrieving excursion data from various sources and 3rd parties, examining steps in the supply chain that went wrong and processing the paperwork to finally determine a month later the shipment is ok to be used. In the meantime, has the manufacturer re-shipped new stock, or have clinical trials been delayed while all this manual processing work was going on?
Industry benchmarking approach
A group of like-minded professionals faced with the same temperature control distribution challenges got together at the 2015 Leading Minds Seminar. During the two days, Stability Budget became an ongoing discussion. Practitioners exchanged ideas on how a central database can be used to collect and communicate data to support distribution, having all the information in one place to answer excursions questions immediately and track excursions across the supply chain.
The hot topic discussion was called “Community Forum Discussion: Stability Budget Approaches and their Benefits”. Merck, AbbVie and ELPRO took part in the discussion. Below is the Questions & Answers from the discussion.
What’s the difference between a stability budget and TOR (Time out of Refrigeration)?
Most agree they are fairly synonymous terms. Some believe TOR means for just within manufacturing, but once shipped it’s a stability budget. TOR is typically formally tracked within a manufacturer’s operations.
Literally, each time a product is placed into and then removed from refrigerated storage, the time is recorded and Time Out of Refrigeration is tracked. Perhaps the most common use of the term TOR is the spent portion of the established Stability Budget (Stability Budget – TOR = Remaining Stability Budget (RSB)).
Time out of Controlled Storage, Time out of Freezer, Time out of Storage, are all terms used. There are discussions currently between some large pharma what the standardized language will be.
How do you build a stability budget?
Each clinical (often less available data) and commercial products will have upper and lower limits defined that a product is stable for. These parameters are set by your development team during ICH stability testing. There are various ways to implement a SB, but the basic principle is to allocate a portion of the stability budget to each ‘leg’ of the supply chain. By tracking how much stability is ‘used up’ in each leg, you know your total Time out of Refrigeration, or how much stability budget you have left.
A budget must always include some allowance for patient ‘leg’ of chain, and ‘extra bucket’ for contingency.
Possible breakdown of buckets for the Stability Budget include:
-
Manufacturing (+9 °C..+25 °C)
-
Packaging (+9 °C..+25 °C)
-
Warehouse operations (+9 °C..+25 °C)
-
Shipping (defined by data)
-
User (+9 °C..+25 °C)
-
Excursions (defined by data)
Each of these buckets then are given an allowance. See image 1.1 below for an example of a stability budget.
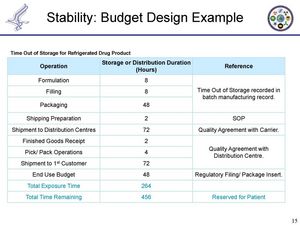
How do you track a stability budget through the product lifestyle?
There are several ways to manage or track a stability budget.
One approach can be ‘manual’, where each leg is assessed for deviations and one person is responsible for asking handler what budget is left and ‘adding up’ the deviations.
A ‘distribution’ approach, retrieves any unused stability budget from previous leg to use for future legs. However you can never borrow budget from future legs.
A more advanced, automated approach is ‘Push Forward Remaining Stability Budget (RSB)’. With the first two approaches above, you can manually collect the budget from previous handler to program the subsequent or next shipment data logger. With the more advanced approach ‘RSB’ and aided by an ERP integration, the subsequent shipment’s data loggers would be programmed using a ‘batch record’ number from the ERP system which would be tracking the budget along the entire lifecycle, subtracting used budget.
When is a stability budget available, and to whom?
Like most temperature data initiatives, stability budget has to be created by and is owned by the product manufacturer to ensure no tampering, changing etc of data. For some manufacturers, QA retains control of the budget, allowing supply chain partners or customers access to only their allowed portion of the budget that they must maintain and control while in their responsibility.
Some supply chain departments have had challenges in getting all the stability budget from the manufacturing team or department, can be difficult if on another continent for example. Best advice here to keep asking, to find the right person within the manufacturing organization, or go back to Pharma Development.
Stability Budgets – accelerated stability testing – is conducted during product development by the manufacturer.
Who creates a stability budget?
Different organizations had different responses, Analytical department, QA, Product quality, different approval levels.
Which department who manages a stability budget?
Those with SB experience were adamant the data comes from the Development team, but QA always manages it on-going in the supply chain.
Does a budget account for regional differences?
Yes it can.QA and Logistics together need to decide if more allowance should be given during certain more challenging profiles will be used, for example during winter months in the northern hemisphere.
What type/amount of testing is needed?
The Analytical or Development team follow strict ICH 7 guidelines for standard and accelerated stability tests. See also the PDA TR 53
Which products need a stability budget?
All products.
Where does a stability budget end?
At patient consumption or administration. See Stability Budget buckets above.
How do you budget for unknowns?
Most audience and panelists agree there should always be an ‘ooops’ or ‘extra bucket’ to build in realistic contingency to your budget.
What are the benefits of a stability budget?
-
Saves discarded product ($!)
-
Reduces logistics and packaging cost ($!)
-
Transparency in processes = robust
-
Optimize inventory
-
Benefit patients and customers!
-
Reduce documentation and alarms, by using pre-programmed multi-level alarm data logger
The seminar discussion could have continued as there was a lot of interest on this topic. One of the participants said «We need to think more about a comprehensive stability budget. We have temperature and time criteria for shipments that go outside of the labeled storage conditions. We do not have a process to track those excursions in the event they were to happen multiple times throughout the lifecycle of the product.»
Another person commented in the post-event evaluation «Our industry is challenged with new regulations and we need new technology to keep up with operational efficiencies to maintain product integrity and cost factors.»
There are a lot of discussions amongst industry groups regarding stability budget. It’s important to point out there are a lot of recommendations, not one absolute way, to set up a stability budget. The PDA PCCIG Group has written Technical Report 53 that outline steps for implementing a stability budget that is used as helpful guidance.
At this point, for most companies using a stability budget for each product is too new of a concept. Pharmaceutical manufacturers need to apply existing ‘manual’ and ‘distribution’ approaches to their products to start the ball rolling; and hopefully one day we will be using the ‘Push Forward the Remaining Stability Budget Approach’ across the industry to ensure efficient, automated decisions for product release and quality control.
The other important question to ask yourself, without using a stability budget, how can you guarantee to your patients that their medicine is safe to consume?
Visit leading-minds-network/seminars to find out when the next Leading Minds Seminar will take place in your area to take part in discussions with leading industry representatives.







Leave a Comment